Hepatitis C Blog
Greg Jefferys Hepatitis C blog deals with all the issues associated with hepatitis C
The Best Hepatitis C Treatment
- March 7, 2019
- By Greg Jefferys
- 11 Comments
What is the Best Treatment for Hepatitis C?
Those of you who read my blog posts on a regular basis will know that for a long time I have been “banging the drum ” about Sofosbuvir + Daclatasvir being the best Hepatitis C treatment.
Better than Epclusa, better than Mavyret, better than Harvoni AND much cheaper than all of them.
A recent study published only a few weeks ago in the Journal of Hepatology proves again that Sofosbuvir 400 mg + Daclatasvir 60 mg, as well as being the best treatment for genotype 1, Sof + Dac is also is the best treatment for Hepatitis C genotype 2 and genotype 3.
Adding Ribavirin
Importantly this study shows that Sofosbuvir + Daclatasvir is equally effective with or without Ribavirin.
In fact adding Ribavirin appears to REDUCE the cure rate!!!!
In other words it shows that there is no point in including Ribavirin when treating Hep C G2 or G3.
This is very important because Ribavirin is very toxic and (particularly for women) can produce serious side effects.
The Best and the Cheapest
The fact is that the best Hepatitis C treatment is also the cheapest. Strangely it is also the treatment least likely to be prescribed in Western Countries such as the USA, Canada and Europe. The reason for this is that BIG Pharma does not make any money for Sofosbuvir + Daclatasvir because the patent for Sofosbuvir is owned by Gilead and the patent for Daclatasvir is owned by BMS and the two Big Pharma giants could not come to an agreement on cooperation.
So it happens that the best and cheapest treatment for Hepatitis C is not being used in many countries simply because Big Pharma has let its greed block what is best for people with Hepatitis C
Here is the Abstract of the research results:
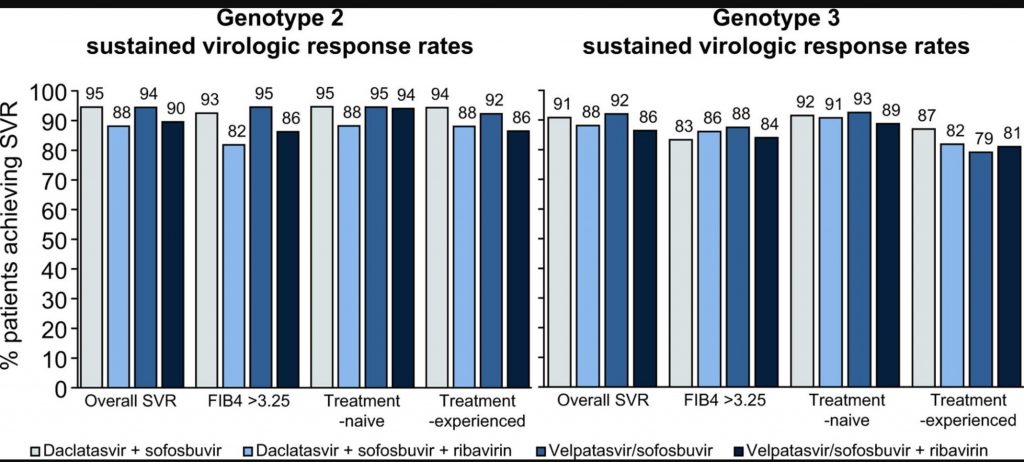
This graph shows that there is no significant difference in cure rates between Sofosbuvir + Velaptasvir (Epclusa) and Sofosbuvir + Daclatasvir. The exception is where liver cirrhosis is present, in which case Sof + Vel is slightly superior and where a patient has failed previous treatment, where Sof + Dac is significantly superior
J Hepatol. 2019 Jan;70(1):15-23. doi: 10.1016/j.jhep.2018.09.018. Epub 2018 Sep 26.
Real-world effectiveness of Daclatasvir/Sofosbuvir and Velpatasvir/Sofosbuvir in hepatitis C genotype 2 and 3.
Belperio PS1, Shahoumian TA1, Loomis TP1, Mole LA1, Backus LI2.
BACKGROUND & AIM:
Understanding the real-world effectiveness of all-oral hepatitis C virus (HCV) regimens informs treatment decisions. We evaluated the effectiveness of daclatasvir + sofosbuvir ± ribavirin (DCV + SOF ± RBV) and velpatasvir/sofosbuvir (VEL/SOF) ± RBV in patients with genotype 2 and genotype 3 infection treated in routine practice.
METHODS:
This observational analysis was carried out in an intent-to-treat cohort of patients with HCV genotype 2 and genotype 3. Sustained virologic response (SVR) analysis was performed in 5,400 patients initiated on DCV + SOF ± RBV or VEL/SOF ± RBV at any Department of Veterans Affairs facility.
RESULTS:
For genotype 2, SVR rates did not differ between DCV + SOF (94.5%) and VEL/SOF (94.4%) or between DCV + SOF + RBV (88.1%) and VEL/SOF + RBV (89.5%). For genotype 3, SVR rates did not differ between DCV + SOF (90.8%) and VEL/SOF (92.0%) or between DCV + SOF + RBV (88.1%) and VEL/SOF + RBV (86.4%). In multivariate models of patients with genotype 2 and 3 infection, the treatment regimen was not a significant predictor of the odds of SVR. For genotype 3, significant predictors of reduced odds of SVR were prior HCV treatment-experience (odds ratio [OR] 0.51, 95% CI 0.36-0.72; p <0.001), FIB-4 >3.25 (OR 0.60; 95%CI 0.43-0.84; p = 0.002) and a history of decompensated liver disease (OR 0.68; 95%CI 0.47-0.98; p = 0.04). For patients with genotype 2 and 3, treated with VEL/SOF ± RBV, 89% and 85% received 12-weeks of treatment, respectively. For DCV + SOF ± RBV, 56% and 20% of patients with HCV genotype 2 received 12-weeks and 24-weeks of treatment, respectively; while 53% and 23% of patients with HCV genotype 3 received 12-weeks and 24-weeks, with most direct-acting antiviral experienced patients receiving 24-weeks.
CONCLUSIONS:
In patients infected with HCV genotype 2 and 3, DCV + SOF ± RBV and VEL/SOF ± RBV produced similar SVR rates within each genotype, and the regimen did not have a significant impact on the odds of SVR. For patients with genotype 3, prior treatment-experience and advanced liver disease were significant predictors of reduced odds of SVR regardless of regimen.
LAY SUMMARY:
In clinical practice, cure rates for hepatitis C virus (HCV) genotype 2 were 94% and cure rates for HCV genotype 3 were 90%. The chance of achieving cure was the same whether a person received daclatasvir plus sofosbuvir or velpatasvir/sofosbuvir. Ribavirin did not affect cure rates. The chance of a cure was lowest in people who had received HCV medication in the past.
Published by Elsevier B.V.
Hepatitis C Treatment Times
It is important to remember that the results above are for 12 weeks treatment. By increasing the treatment time the cure rate will improve significantly. Below is a rough scale showing how increasing treatment time improves cure rate percentages. For more information on Hepatitis C Treatment times please click this link
4 Weeks <50%
12 Weeks 90%>
16 Weeks 95%> (+5%)
20 Weeks 97%> (+2%)
24 Weeks 98%> (+1%)


KEYWORDS:
Daclatasvir; Hepatitis C; Sofosbuvir; Sustained virologic response; Velpatasvir
The study can be found on this link to the Journal of Hepatology 2019;70(1):
Cure For Hepatitis C Genotype 3, Hep C Genotype 3 Cirrhosis, Hep C Genotype 3 Cure, Hep C Genotype 3 Cure Rate, Hep C Genotype 3 Uk, Hepatitis C Genotype 3, Hepatitis C Genotype 3 A, Hepatitis C Genotype 3 Causes, Hepatitis C Genotype 3 Cirrhosis Treatment, Hepatitis C Genotype 3 Resistance Testing, Hepatitis C Genotype 3 Symptoms, Hepatitis C Genotype 3 Transmission, Hepatitis C Genotype 3 Treatment, Hepatitis C Genotype 3 Treatment In India, Is Hepatitis C Genotype 3 Curable
- Post Categories
- Uncategorized
Greg Jefferys
Tags
- Cure For Hepatitis C Genotype 3
- Hep C Genotype 3 Cirrhosis
- Hep C Genotype 3 Cure
- Hep C Genotype 3 Cure Rate
- Hep C Genotype 3 Uk
- Hepatitis C Genotype 3
- Hepatitis C Genotype 3 A
- Hepatitis C Genotype 3 Causes
- Hepatitis C Genotype 3 Cirrhosis Treatment
- Hepatitis C Genotype 3 Resistance Testing
- Hepatitis C Genotype 3 Symptoms
- Hepatitis C Genotype 3 Transmission
- Hepatitis C Genotype 3 Treatment
- Hepatitis C Genotype 3 Treatment In India
- Is Hepatitis C Genotype 3 Curable
11 Comments
Leave a Comment Cancel Reply
Join my Hep C Support Group.
Talk privately to other people
with Hep C in a closed group.
Disclaimer
Greg Jefferys’ blog is provided for informational purposes and is not intended as Medical advice, diagnosis, or treatment.
Whilst Greg Jefferys is doing a PhD it is not in medicine. Any advice offered is offered in good faith and based on an extensive general knowledge of Hepatitis C and access to generic Hepatitis medicines Greg Jefferys has acquired through his work as an advocate and activist
The Hep C Buyers Club is not a company or corporate entity but simply a loose structure intended to offer a free information to people with Hepatitis C
Other Books
Click here for other books by Greg Jefferys.
for Kindle
I have converted this diary into a kindle book for folk who might like it in that format. I have added a lot more depth than the original diary contains, it’s more of a complete story in book format. I have priced it as low as Kindle allows me to @ 99 cents. If you are interested just click here to go to the Kindle page.
Recent Posts
-
Hepatitis C Treatment and Liver Cancer June 19, 2024
-
Motivate C a Profitable Hep C Initiative April 29, 2024
-
After Hep C Healthy Liver Diet September 4, 2023
-
Fear of Hep C Treatment April 14, 2023
-
My Letter to Joe Biden March 2, 2023
-
Dormant Hepatitis C November 27, 2022
-
How Much water to drink during Hep C treatment October 13, 2022
-
Hep C and Peripheral Neuropathy August 21, 2022
-
Fatigue Brain Fog and Hepatitis C August 18, 2022
-
Hep C and Liver Cancer May 18, 2022
Contact Us
If you have any questions please reach out by email, or complete the below form.
Greg Jefferys3439 Channel Highway, Woodbridge, Tasmania, 7161.
Email: gregjefferys@outlook.com




I have the 550 greg and i am ready to do once and for all.
Hi my name is Daniel I don’t have a lot of money and can’t afford insurance I’ve had this for awhile. I take care of my family and do I work but most of my money and my time go to bills and my 4 children. I’m trying to live and watch my kids grow up I’m not asking to get it for free I’m asking for a affordable price so I can finally be free of this disease. Please and thank you for taking your time in reading this
HI Daniel
If you could email me I will certainly do whatever I can to help you.
I look forward to hearing from you
Greg
Hi, I have a relative that was diagnose with hepatitis C . Is medicine expensive in Mexico?
The price is US$550 for a 12 week treatment
My friend was diagnosed of it two weeks ago. He has been find it difficult to get the drug here in Nigeria. Could u be of help
Please just write to me by email… I tried to reply to the email that you left here but it bounced back
Para es mexico
No es un problema enviarle estos medicamentos a México. Envío medicamentos a la hepatitis c a México casi todas las semanas.
My lousy insurance only pays for 12 weeks of Epclusa which I started 4 days ago for Hep C Geno 3 fibrosis stage 3.
Sadly … Its just about the money with most health insurance companies